Which Coating Should I Use to Protect Neodymium Magnets?
Neodymium magnets are known for their powerful magnetic properties, with the title of “King of Permanent Magnets”. They are widely used in various fields, including automotive, wind power generation, digital electronics, medical equipment etc.. However, they are easily to be corroded and oxidized. So at reality we usually process surface treatment to the final products to prolong its life and protect the performance.
1. Why Does NdFeB Magnet Have to Be Protected by Coating?
NdFeB magnet likes a biscuit with many holes and the inner very active, and the coating is its protective clothing. If not protected, the magnet will quickly rust and oxidize when exposed to air.
That will lead to:
- Appearance Rust: Powder or rust spots appear on the surface, influencing the appearance.
- Performance Decrease: Rust will extend from the surface of the magnet to the interior, causing an irreversible decline in magnetic properties.
- Pulverization and Flaking: Severe corrosion can cause the magnet to crack and ultimately fail.
Therefore, by electroplating, epoxy spraying, a dense protective layer will cover the surface of magnet to isolate it from air and water. This is a key step to ensure the long-term stable operation of NdFeB magnet.

2. Coating Types
There are many types of coatings for NdFeB magnet. The most common ones are the three types: Metal coatings ,Organic coatings and Chemical conversion coatings.

(I)Metal Coatings
A thin metal film is formed on the magnet surface by electroplating.
- Zinc (Zn) Plating: It is chemically more active than iron. In corrosive environments, it corrodes before the iron, thus protecting the inner magnets. Even scratches in the coating still provide protection. Zinc plating is typically followed by a passivization treatment, which forms a dense passive film on the surface, significantly increasing its rust resistance.
- Nickel (Ni) Plating: The most common Nickel plating is Ni-Cu-Ni. The bottom layer of nickel provides excellent bonding; the middle copper layer perfectly covers the surface micro-hole of magnet; the external layer of nickel provides bright appearance, high hardness and wear resistance.
- Gold (Au) Plating: Gold is characterized by its corrosion-resistant, electrically conductive, and solderable. Due to its high cost, it is primarily used in high-reliability electronic components, such as aerospace, military, and high-end medical.
- Silver (Ag) Plating: Silver has the best electrical and thermal conductivity of all metals. Its disadvantage is that it easily oxidizes and turns black, which affects soldering and electrical conductivity. It is mainly used in special applications requiring high-frequency and radio frequency conductivity.
- Tin (Sn) Plating: Provides barrier protection and excellent solderability. Tin plating is soft, has good ductility, and excellent solderability. It is environmentally friendly and is often used on pins and terminals of electronic components to prevent oxidation and ensure reliable soldering. Its disadvantages are that it converts to powder at low temperatures and has poor wear resistance.
(II)Organic Coatings
A polymer coating of organic compounds is applied to the magnet surface by a physical process. It provides a comprehensive barrier, unlike electroplating, which carries the risk of electrochemical corrosion. It is the first choice for extreme environments.
- Epoxy: It is the thickest barrier protection. A uniform, dense, and non-porous polymer coating is formed on the magnet surface by electrostatic spraying.
- Electrophoretic Coating (E-Coat): Similar to epoxy coating, but with a different process. The work-piece is immersed in a charged coating solution, where the electric field forces the coating particles to be evenly deposited onto the surface. The coating is then cured by baking.
- Parylene Coating: A polymer coating deposited by vacuum vapor deposition. Its raw material (dimer) is vaporized and decomposed into active monomers in a vacuum environment. These monomers then spontaneously polymerize on the work-piece surface to form a completely covered polymer film.
(III)Chemical Conversion Coatings
Chemical conversion coatings: Chemical/electrochemical reactions are used to create a self-adhering, insoluble compound film on the metal surface.
Phosphating is a process in which a porous, insoluble phosphate crystal film is formed on the surface of steel through a chemical reaction. The essential difference between this coating and all other coatings is that the phosphate film is a natural conversion of the base metal, not an externally deposited layer. It appears gray or dark gray and has a rough surface.
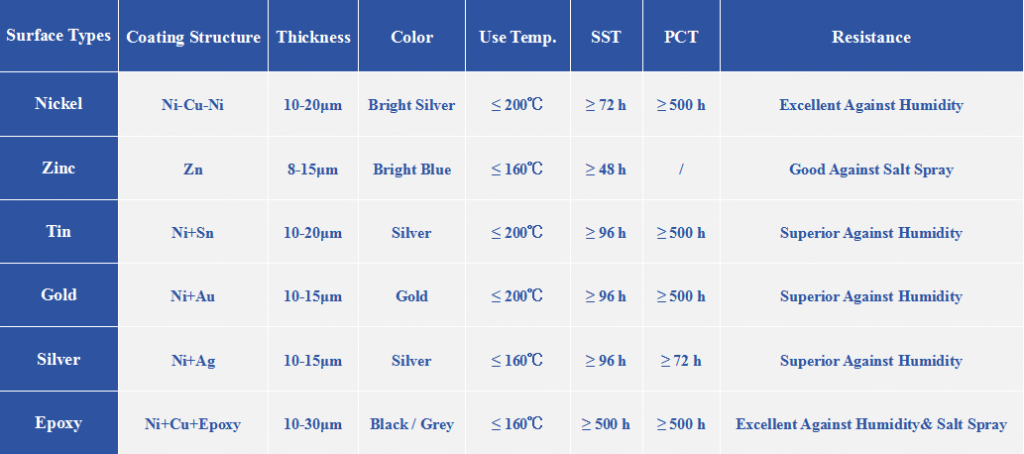
3. How to choose coatings?
When choosing the coating, we should consider the following factors:
- Usage environment: Dry indoor? Humid outdoor? Corrosive environment?
- Cost budget: Strict budget constraints?
- Appearance: Bright appearance?
- Dimensional accuracy: Strict coating thickness?
- Special requirements: Insulation?Contact with other metal? Solderability?
Based on practical application and experience, you can consider the following when choosing coatings:
- Low cost & Internal use: Zinc Coating
- Good appearance & Wear resistance: Nickel Coating
- Extreme environment & Optimal protection: Epoxy Coating
- Special features (conductivity, solderability): Gold / Silver / Tin Coating
- Highest reliability & regardless of cost: Parylene Coating
4. Still unsure? Our team will help you.
Choosing coating is a technical decision which comprehensively consider the environment, cost, appearance, functionality. If you still have question about the best choice for specific application, our technical team is ready to provide you free and professional consultation.
